Hi all qPCR users (or not),
Just few words to thank all visitors to come and read me because it encourages me to carry on.
To be honest, I do a french version for few years and I decided to translate it for non-french speaking people.
Actually, I have started it because when I wanted to find informations about qPCR, I found few tutorials, forums of course, but almost nothing for troubleshooting, special cases or tips to know about the technique.
And even if you are not thousands of people reading my work, you can be up to hundreds per month to come on my blog.
So I will try to carry on translation and update as fast as possible, but do not hesitate as well to send me questions or comments to make this blog even more interactive.
I write actually about inhibition control and how to handle it with commercial kits but also with personal construction for competitive control to mimic as close as possible target system.
Thanks to everyone and see you soon.
mardi 16 octobre 2012
mercredi 3 octobre 2012
Probe strategy : DIY
When the work is done with primers and SybrGreen meaning that PCR answer is good comprising a nice specificity validated with melting curves (single peaks, no shoulders, no close primer-dimer from targeted melting temperatures..), next step is to add a probe to the PCR system and to improve one more time your PCR system.
But talking about probes lead also to talk about different strategies :
First, probes has to be grafted with fluorophores. These molecules has to be wisely chosen depending on your final strategy : simplex (only one target per well) or multiplex (from 2 to 4 different fluorophores in the same well). 4-pex is pretty rare for quantification, we can find some more in genotyping detection.
For example, Eurogentec and Molecular Probes websites provide good table to compare fluorophores spectra you can use for qPCR probes.
In most of the possible strategies about qPCR, there are 2 different molecules called Reporter (fluorescent molecule) and Quencher (molecule that can absorb light energy emitted by Reporter when close enough and transform it into heat). That is by the way, the FRET principle...
When Reporter is excited by a light source, it emits a light but is also immediately quenched and emitted through heat. When the probe is degraded through PCR process, the Reporter is released from the probe, get far from the Quencher and can at least be seen by detection system.
This leads to the following next technology :
TaqMan(R) probe : single oligonucleotide grafted in 5' by the Reporter and in 3' by the Quencher.
This technology relies on Taq polymerase specificity : an exonucleasic activity during complementary strand transcription. Thus, when the probe hybridize on target sequence, there is still no fluorecence detected because Quencher molecule is still close to Reporter. Because reporter is released in the solution due to Taq exonucleasic actitivity and get far from quencher, fluorescence can then be detected.
This method is the most used in qPCR.
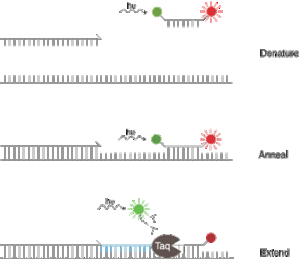
"FRET" probes : used mainly by Roche. Actually, this comprises 2 oligonucleotides hybridizing close together. Both are is grafted with one molecule, one is called "donor" and the other one "acceptor". (cf hereunder). Probes place is crucial for a good fluorescence answer. In this method, the two probes are essential to get a signal. When they are well hybridized, they are close enough to operate an energy transfer between them. The donor get the energy and transfer it to acceptor one. Fluorescence will then be detected from this step.

Molecular Beacon (MB) :
Another probe type developed by Kramer team in New-York in 1999 (http://www.molecular-beacons.org/). And because their website is very nice, I will not try to explain MB better than them.
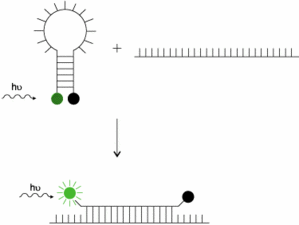
MB design is once again crucial for this specific probe strategie but still using the FRET system for energy transfer. As can be seen on next figure, the probe is grafted with a Reporter (green) and a quencher (black). Its very special hairpin structure needs a very specific design in order to get a specific hybridization without interfering with target hybridization.
When target sequence hybridizes the probe, this one unfold completely to the sequence. In the meantime, Reporter gets further from the Quencher and fluorescence can occur and be detected. This strategy does not imply hydrolysis of the probe by the Taq and can be used again during nect amplification step.
This strategy work very well and is used especially for genotyping. Fluorescence has to be detected during hybridization step and not elongation if you use 3-step temperature for PCR reaction.
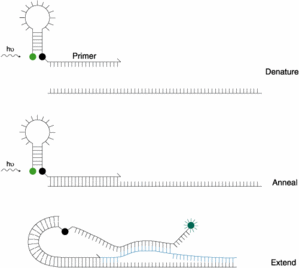
Scorpions :
This strategy is more primers modification rather than probe strategy. Somewhere, it is a mix between a MB probe grafted on a primer (see figuer hereunder). Very few references about the use of scorpions primers even if part of many oligo supplier catalog.
Few other strategies exist for qPCR application like Uniprimers, but they are not so used in routine application.
In a next post, I will talk about fluorophore strategies that can be used on probes.
Have good amplification and see you next time...
But talking about probes lead also to talk about different strategies :
First, probes has to be grafted with fluorophores. These molecules has to be wisely chosen depending on your final strategy : simplex (only one target per well) or multiplex (from 2 to 4 different fluorophores in the same well). 4-pex is pretty rare for quantification, we can find some more in genotyping detection.
For example, Eurogentec and Molecular Probes websites provide good table to compare fluorophores spectra you can use for qPCR probes.
In most of the possible strategies about qPCR, there are 2 different molecules called Reporter (fluorescent molecule) and Quencher (molecule that can absorb light energy emitted by Reporter when close enough and transform it into heat). That is by the way, the FRET principle...
When Reporter is excited by a light source, it emits a light but is also immediately quenched and emitted through heat. When the probe is degraded through PCR process, the Reporter is released from the probe, get far from the Quencher and can at least be seen by detection system.
This leads to the following next technology :
TaqMan(R) probe : single oligonucleotide grafted in 5' by the Reporter and in 3' by the Quencher.
This technology relies on Taq polymerase specificity : an exonucleasic activity during complementary strand transcription. Thus, when the probe hybridize on target sequence, there is still no fluorecence detected because Quencher molecule is still close to Reporter. Because reporter is released in the solution due to Taq exonucleasic actitivity and get far from quencher, fluorescence can then be detected.
This method is the most used in qPCR.

"FRET" probes : used mainly by Roche. Actually, this comprises 2 oligonucleotides hybridizing close together. Both are is grafted with one molecule, one is called "donor" and the other one "acceptor". (cf hereunder). Probes place is crucial for a good fluorescence answer. In this method, the two probes are essential to get a signal. When they are well hybridized, they are close enough to operate an energy transfer between them. The donor get the energy and transfer it to acceptor one. Fluorescence will then be detected from this step.

Molecular Beacon (MB) :
Another probe type developed by Kramer team in New-York in 1999 (http://www.molecular-beacons.org/). And because their website is very nice, I will not try to explain MB better than them.
MB design is once again crucial for this specific probe strategie but still using the FRET system for energy transfer. As can be seen on next figure, the probe is grafted with a Reporter (green) and a quencher (black). Its very special hairpin structure needs a very specific design in order to get a specific hybridization without interfering with target hybridization.
When target sequence hybridizes the probe, this one unfold completely to the sequence. In the meantime, Reporter gets further from the Quencher and fluorescence can occur and be detected. This strategy does not imply hydrolysis of the probe by the Taq and can be used again during nect amplification step.
This strategy work very well and is used especially for genotyping. Fluorescence has to be detected during hybridization step and not elongation if you use 3-step temperature for PCR reaction.

Scorpions :
This strategy is more primers modification rather than probe strategy. Somewhere, it is a mix between a MB probe grafted on a primer (see figuer hereunder). Very few references about the use of scorpions primers even if part of many oligo supplier catalog.

Few other strategies exist for qPCR application like Uniprimers, but they are not so used in routine application.
In a next post, I will talk about fluorophore strategies that can be used on probes.
Have good amplification and see you next time...
Inscription à :
Commentaires (Atom)